In 2019, McKinsey Global Institute’s team began researching the huge potential that developments on the biotechnology front held out for the world. To do that, laboratories began working on sequencing of genomes.
Coincidentally, this research suddenly found immense relevance when the COVID-19 pandemic hit the world. Suddenly, everyone began to realise that “Sequencing is just the start: bio-innovations are enabling the rapid introduction of clinical trials of vaccines, the search for effective therapies, and a deep investigation of both the origins and the transmission patterns of the virus,” says the McKinsey report on The Bio-Revolution (April 2020).
This new technology currently harnesses all the big forces that have enamoured the business community -- big data, the Internet of Things (IoT), automation, and Artificial Intelligence (AI). Together, they are bound to have a huge impact on public health and healthcare systems, pharmaceuticals, and medical products.
And yet, like always, India has been caught napping. Even its lead institutes – ICMR (Indian Council of Medical Research) and NCDC (National Centre for Disease Control) – are at war with each other, trying to seek out relevance. This is primarily because the government itself isn’t clear which responsibilities should be taken up by which organisation, and whether it makes sense even to have two organisations instead of just one.
As a media report points out, NCDC has had its share of conflicts with the state governments in collecting data on outbreaks. Even ICMR has not been professional in its approach towards the industry. Remember the quixotic manner in which it tried to set a deadline for finding a vaccine for coronavirus by August 15, 2020?
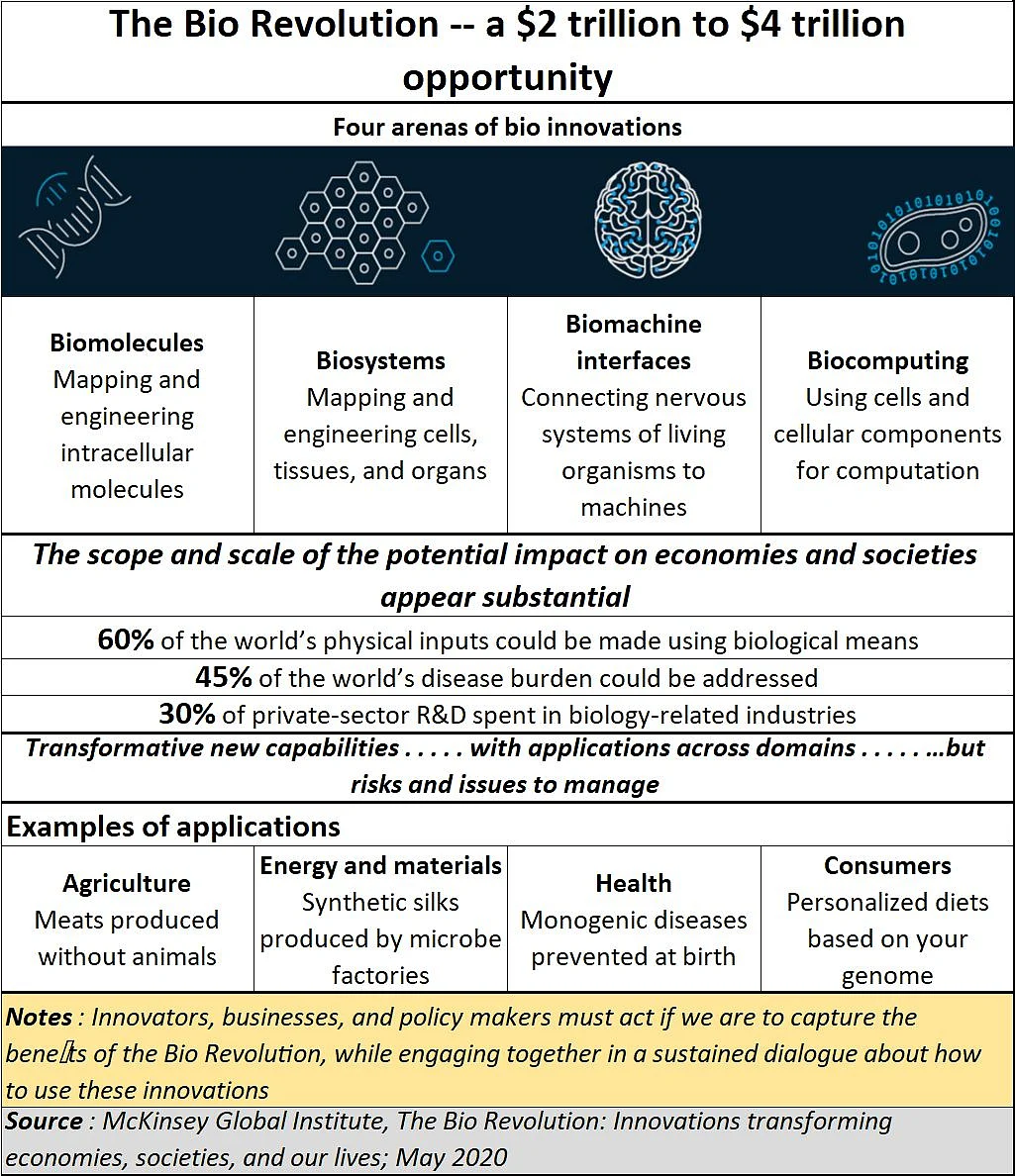
If India must even attempt to grab a share of the $4 trillion market for bio-disrupters in the marketplace, it has to look at creating policies in at least four areas. And this cannot be done by tinkering with older policies, or applying a band-aid over the loopholes existing policies have.
It needs to begin with education – where the best brains (without reservations please) are allowed to study molecular sciences, biotechnology and medicine at the best Indian Institutes.
This also means attracting the best teachers in each of the related disciplines. Hence policies relating to recruitment (once again, no reservations) and more attractive emoluments need to be put into place. Since many of these professionals would not be happy to be working full-time in Universities and research centres, some sort of accommodation will have to be made, so that the best brains in industry are allowed (once again, most state governments drove them away) to teach and train a fresh crop of students year after year.
The third area which will have to be addressed is the legal system. Policies will have to be created to attract, or integrate, professionals from these fields to handle disputes relating to safety, intellectual property rights, and standards. The scope of judges, and the manner in which the judiciary can co-opt such professionals, will also have to be examined and expanded.
The fourth area of policy reform will relate to governance. Much of the work that existing departments like the ICMR and the FDA (Food and Drugs Administration) do is not based on solid scientific study. A good example is the manner in which an associate body of the FDA, the FSSAI, chose to ban the products of Nestle. Finally, the matter went to the courts which asked FSSAI to desist from banning Nestle’s Maggi noodles. A country cannot move into higher levels of science and biotechnology if its administrative officers are not competent to understand, or pass judgements on, the issues involved. This means revamping the quality of officers appointed, and swift punishments for people who take decisions without proper study of the underlying issues.
If the four issues dwelt with above cannot be addressed, India can kiss goodbye to the huge market that will emerge. Bye-bye also to the prime minister’s exhortation for an Aatma Nirbhar Bharat.
As the chart alongside shows, success in this market will depend on a country’s “increasing ability to understand and engineer biology,” says the McKinsey report. This includes sequencing DNA affordably and effectively, and mastering new techniques (including CRISPR) to edit genes and reprogram cells. This is bound to involve cross-disciplinary approaches to biomolecules (mapping , measuring, and engineering of molecules), bio-systems (engineering of cells, tissues, and organs), bio machines (interfaces between biology and machines) and biocomputing (using cells or molecules such as DNA for computation).
If India manages these well, it could address more effectively issues relating to food, medicine, disease control and even future application in the biological world. All these will become extremely important when it comes to automation, machine learning, and enhancing the capability of biological behaviour. They could also enhance research into ways to restore sensory function to the brain, and using biocomputers that use DNA to store data.
As the McKenzie report states, “It is possible that bio innovations could impact up to 60 percent of physical inputs.. . . . [and] at least 45 per cent of the current global disease burden could be addressed using science that is conceivable today.”
But the first decision will always revolve around a crucial political and social issue. Is the government ready to give precedence to merit? Or will it prefer to blunder through self-created hurdles of protection and incompetence?
These decisions must be taken now. The government cannot kick the can down the road any further.
The author is consulting editor with FPJ










